Multiple Choice
Copper has an atomic number of 29 and a mass number of 64. What would result if an uncharged copper atom lost two electrons?
5002
views
 Verified step by step guidance
Verified step by step guidance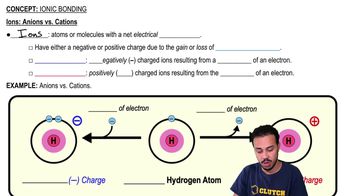



 4:34m
4:34mMaster Ions: Anions vs. Cations with a bite sized video explanation from Bruce Bryan
Start learning