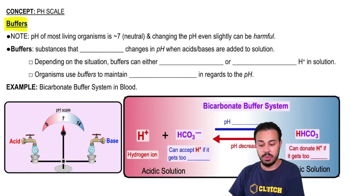
Two substances secreted into the proximal convoluted tubules in exchange for sodium ions are:
a. Ammonium ions
b. Bicarbonate
c. Calcium
d. Chloride
e. Hydrogen ions
f. Magnesium
g. Phosphate
h. Potassium
i. Sodium
j. Water
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: