Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidizing Agent
An oxidizing agent is a substance that gains electrons in a chemical reaction and, in the process, causes another substance to be oxidized. It is reduced itself. In the context of redox reactions, identifying the oxidizing agent involves determining which reactant is reduced by accepting electrons.
Recommended video:
Redox Reactions
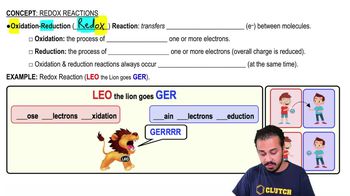
Redox reactions, or oxidation-reduction reactions, involve the transfer of electrons between two substances. One substance is oxidized (loses electrons), and the other is reduced (gains electrons). Understanding which components are oxidized and reduced is crucial for identifying the oxidizing and reducing agents in a reaction.
Recommended video:
Role of NADH in Cellular Respiration
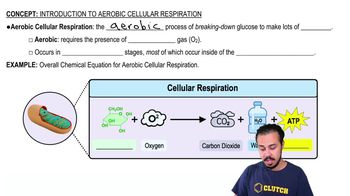
NADH is a coenzyme that plays a critical role in cellular respiration, acting as an electron carrier. It is involved in redox reactions, where it donates electrons and is oxidized to NAD+. In the given reaction, NADH donates electrons to pyruvate, reducing it to lactate, and is itself oxidized, indicating its role as a reducing agent.
Recommended video:
Introduction to Cellular Respiration
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: