Multiple Choice
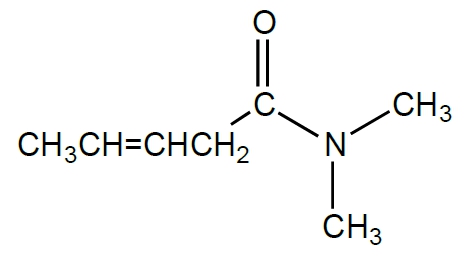
Which functional group is most commonly associated with compounds that have a strong odor, such as those found in garlic and onions?
47
views
 Verified step by step guidance
Verified step by step guidance
 2:14m
2:14mMaster Hydrocarbon Functional Groups with a bite sized video explanation from Jules
Start learning
