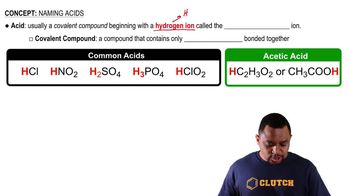
Textbook Question
Give the geometry and approximate bond angles around thecentral atom in CCl3-. (LO 8.1)(a) Trigonal planar, 120°(b) Trigonal pyramidal, 109.5°(c) Trigonal pyramidal, 120°(d) Bent, 109.5°
1296
views

 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 7
Problem 7
 Verified step by step guidance
Verified step by step guidance