The solubility of CaCO3 is pH dependent. (b) Use the Kb expression for the CO32- ion to determine the equilibrium constant for the reaction CaCO3(s) + H2O(l) ⇌ Ca2+(aq) + HCO3-(aq) + OH-(aq)

Calculate the solubility of Mg1OH22 in 0.50 M NH4Cl.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
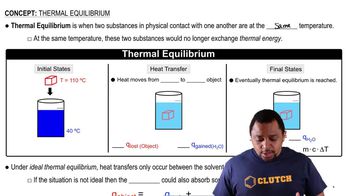
Key Concepts
Solubility Product Constant (Ksp)

Common Ion Effect

Ionic Equilibrium
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca51PO423OH, and whose corresponding Ksp = 6.8 * 10-27. As discussed in the Chemistry and Life box on page 746, fluoride in fluorinated water or in toothpaste reacts with hydroxyapatite to form fluoroapatite, Ca51PO423F, whose Ksp = 1.0 * 10-60. (a) Write the expression for the solubility-constant for hydroxyapatite and for fluoroapatite.
The solubility-product constant for barium permanganate, Ba1MnO422, is 2.5 * 10-10. Assume that solid Ba1MnO422 is in equilibrium with a solution of KMnO4. What concentration of KMnO4 is required to establish a concentration of 2.0 * 10-8 M for the Ba2 + ion in solution?
The solubility product constants of PbSO4 and SrSO4 are 6.3 * 10-7 and 3.2 * 10-7, respectively. What are the values of 3SO4 2 - 4, 3Pb2 + 4, and 3Sr2 + 4 in a solution at equilibrium with both substances?
