Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (b) Can have ml = -1
339
views

 Verified step by step guidance
Verified step by step guidance


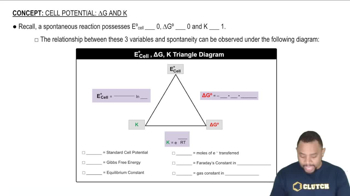
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (b) Can have ml = -1
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (e) Contains the outermost electrons in a beryllium atom
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (f) Can contain two electrons, both with spin ms = +1/2