Textbook Question
An X-ray beam with λ = 154 pm incident on the surface of a crystal produced a maximum reflection at an angle of θ = 28.3°. Assuming n = 1, calculate the separation between layers of atoms in the crystal.
558
views

 Verified step by step guidance
Verified step by step guidance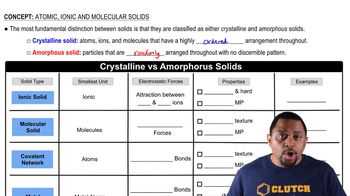


An X-ray beam with λ = 154 pm incident on the surface of a crystal produced a maximum reflection at an angle of θ = 28.3°. Assuming n = 1, calculate the separation between layers of atoms in the crystal.
Determine the number of atoms per unit cell for each metal.
(a) Polonium