How does the molar entropy of a substance change with increasing temperature?

Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. a. NH3(g); Ne(g); SO2(g); CH3CH2OH(g); He(g) c. CH4(g); CF4(g); CCl4(g)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
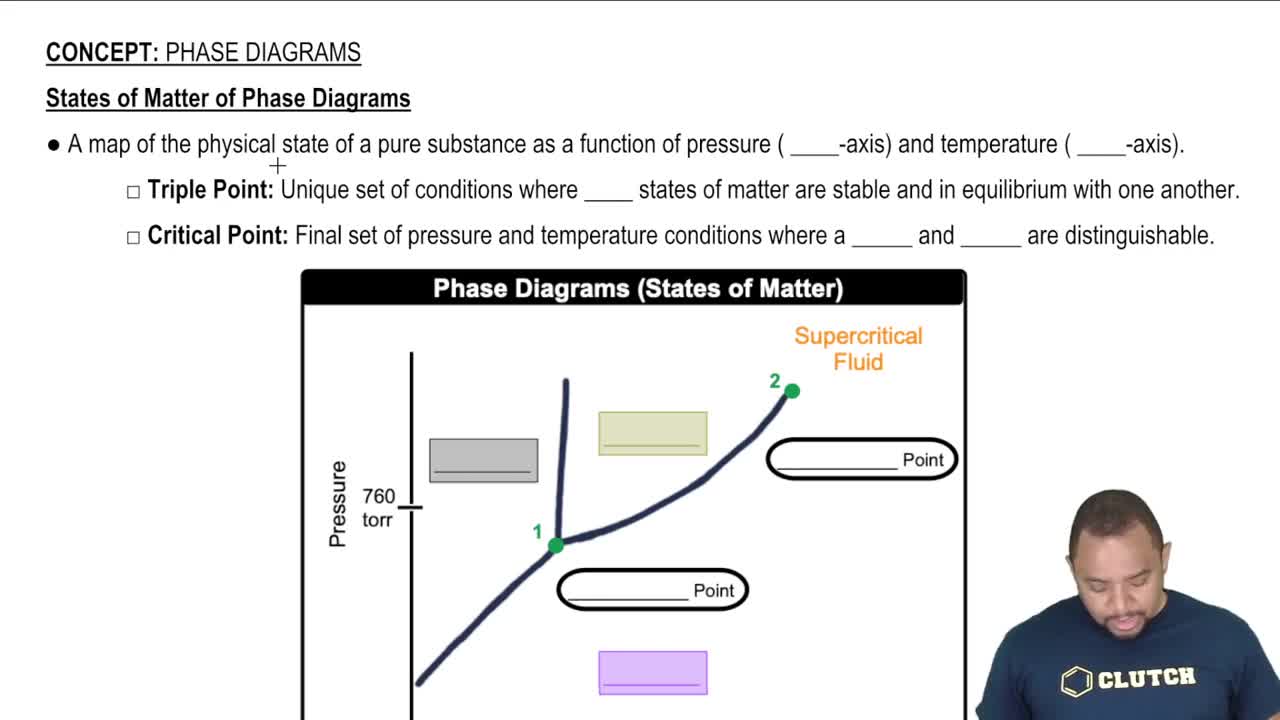
Standard Molar Entropy

Phase of Matter
Molecular Structure and Complexity
For each pair of substances, choose the one that you expect to have the higher standard molar entropy (S°) at 25 °C. Explain your choices. a. CO(g); CO2(g) b. CH3OH(l); CH3OH(g) c. Ar(g); CO2(g) d. CH4(g); SiH4(g) e. NO2(g); CH3CH2CH3(g) f. NaBr(s); NaBr(aq)
For each pair of substances, choose the one that you expect to have the higher standard molar entropy (S°) at 25 °C. Explain your choices. a. NaNO3(s); NaNO3(aq) b. CH4(g); CH3CH3(g) c. Br2(l); Br2(g) d. Br2(g); F2(g) e. PCl3(g); PCl5(g) f. CH3CH2CH2CH3(g); SO2(g)
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. b. H2O(s); H2O(l); H2O(g)
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. a. I2(g); F2(g); Br2(g); Cl2(g) b. H2O(g); H2O2(g); H2S(g) c. C(s, graphite); C(s, diamond); C(s, amorphous)
