Textbook Question
Classify each compound as ionic or molecular. a. CF2Cl2 b. CCl4 c. PtO2 d. SO3
700
views
1
rank


 Verified step by step guidance
Verified step by step guidance


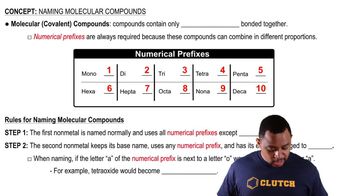
Classify each compound as ionic or molecular. a. CF2Cl2 b. CCl4 c. PtO2 d. SO3
Based on the molecular views, classify each substance as an atomic element, a molecular element, an ionic compound, or a molecular compound.
Write a formula for the ionic compound that forms between each pair of elements. a. silver and chlorine b. sodium and sulfur c. aluminum and sulfur d. potassium and chlorine