Indicate whether each of the following describes a gas, a liquid, or a solid:
a. Lemonade has a definite volume but takes the shape of its container

 Verified step by step guidance
Verified step by step guidance


Indicate whether each of the following describes a gas, a liquid, or a solid:
a. Lemonade has a definite volume but takes the shape of its container
Indicate whether each of the following describes a gas, a liquid, or a solid:
b. The particles in a tank of oxygen are very far apart.
Indicate whether each of the following describes a gas, a liquid, or a solid:
c. Helium occupies the entire volume of a balloon.
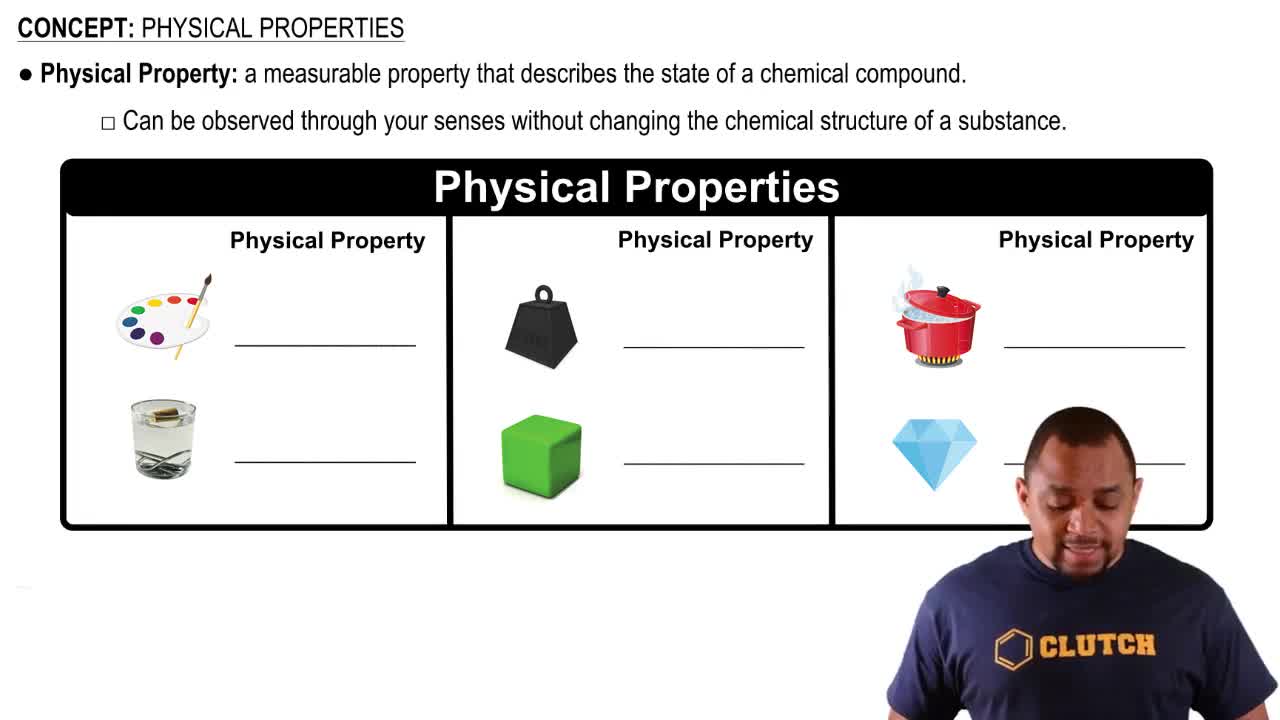
Describe each of the following as a physical or chemical property:
b. Hydrogen reacts readily with oxygen.
Describe each of the following as a physical or chemical property:
a. Neon is a colorless gas at room temperature.
Describe each of the following as a physical or chemical property:
b. Apple slices turn brown when they are exposed to air.