Cyclic ribose nucleotide phosphates, such as cyclic AMP (cAMP), are important signaling agents in living cells; all have the general structure shown here. What kind of linkage holds the phosphate to the ribose (see arrows; ribose is highlighted in blue)?
Ch.17 Carboxylic Acids and Their Derivatives

Chapter 17, Problem 79
Mention at least two simple chemical tests by which you can distinguish between benzaldehyde and benzoic acid.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the chemical difference between benzaldehyde and benzoic acid. Benzaldehyde (C₆H₅CHO) is an aldehyde, while benzoic acid (C₆H₅COOH) is a carboxylic acid. This difference in functional groups will determine the chemical tests to distinguish them.
Step 2: Perform the Fehling's test. Aldehydes like benzaldehyde react with Fehling's solution to produce a red precipitate of copper(I) oxide (Cu₂O), while carboxylic acids like benzoic acid do not react. Add Fehling's solution to each compound and heat gently to observe the result.
Step 3: Conduct the sodium bicarbonate test. Carboxylic acids like benzoic acid react with sodium bicarbonate (NaHCO₃) to produce carbon dioxide gas (CO₂), which can be observed as effervescence. Aldehydes like benzaldehyde do not react with sodium bicarbonate. Add a small amount of NaHCO₃ to each compound and observe for bubbling.
Step 4: Optionally, perform the Tollens' test as an additional confirmation. Aldehydes like benzaldehyde react with Tollens' reagent to form a silver mirror on the test tube, while carboxylic acids like benzoic acid do not react. Add Tollens' reagent to each compound and observe the result.
Step 5: Record and compare the observations from the tests. Benzaldehyde will show a positive result in Fehling's and Tollens' tests but no reaction with sodium bicarbonate, while benzoic acid will show effervescence with sodium bicarbonate but no reaction in Fehling's or Tollens' tests.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Functional Groups
Benzaldehyde and benzoic acid are both aromatic compounds but differ in their functional groups. Benzaldehyde contains an aldehyde group (-CHO), while benzoic acid has a carboxylic acid group (-COOH). Understanding these functional groups is essential for predicting their chemical behavior and reactivity in tests.
Recommended video:
Guided course

Functional Group Priorities Concept 1
Chemical Tests
Chemical tests are specific reactions used to identify the presence of certain functional groups in organic compounds. For instance, the Tollens' test can be used to distinguish benzaldehyde, which can be oxidized to a carboxylic acid, from benzoic acid, which does not react in the same way. Knowing these tests helps in differentiating between compounds based on their chemical properties.
Recommended video:
Guided course
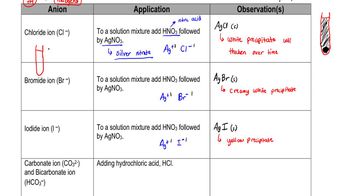
Test for Anions
Solubility and pH
The solubility of benzaldehyde and benzoic acid in water varies due to their functional groups. Benzoic acid is more soluble in water because it can ionize to form benzoate ions, affecting the pH of the solution. This property can be exploited in tests, such as measuring pH changes, to distinguish between the two compounds.
Recommended video:
Guided course

pH and pOH Calculations
Related Practice
Textbook Question
38
views
Textbook Question
What is the difference between a phosphate diester and an ester of a diphosphate? Give an example of each.
58
views
Textbook Question
Propanamide and methyl acetate have about the same molar mass, both are quite soluble in water, and yet the boiling point of propanamide is 213 °C, whereas that of methyl acetate is 57 °C. Explain.
29
views
Textbook Question
Write the formula of the triester formed from glycerol and stearic acid.
905
views
Textbook Question
Each of the following materials has an ester that is responsible for its smell and/or flavor. Search the internet and determine what that ester is, draw its structure, and what carboxylic acid and alcohol are used to form it.
a. Juicy Fruit gum flavoring
628
views
Textbook Question
Draw all possible carboxylic acids with the formula C5H10O2.
587
views
