Textbook Question
Write the names for the elements in each of the following formulas of compounds used in medicine:
a. salt substitute, KCl
796
views

 Verified step by step guidance
Verified step by step guidance

Write the names for the elements in each of the following formulas of compounds used in medicine:
a. salt substitute, KCl
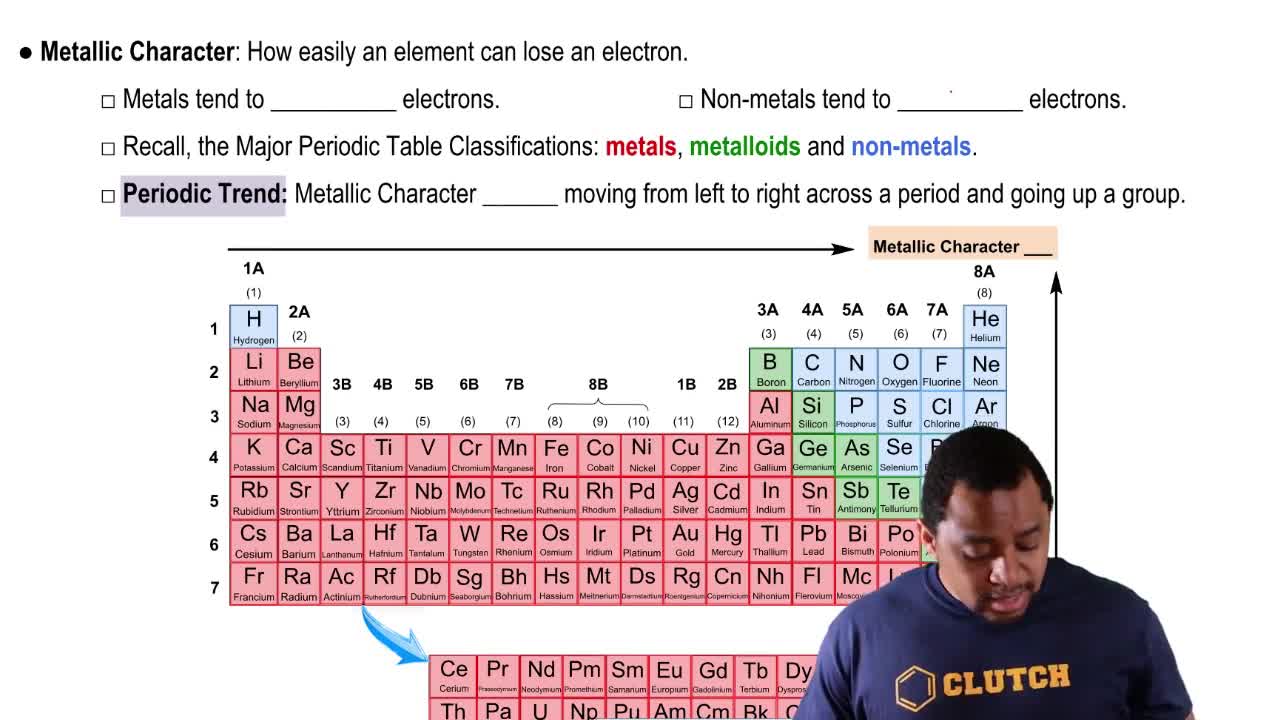
Identify the group or period number described by each of the following:
a. contains C, N, and O
Identify the group or period number described by each of the following:
b. begins with helium
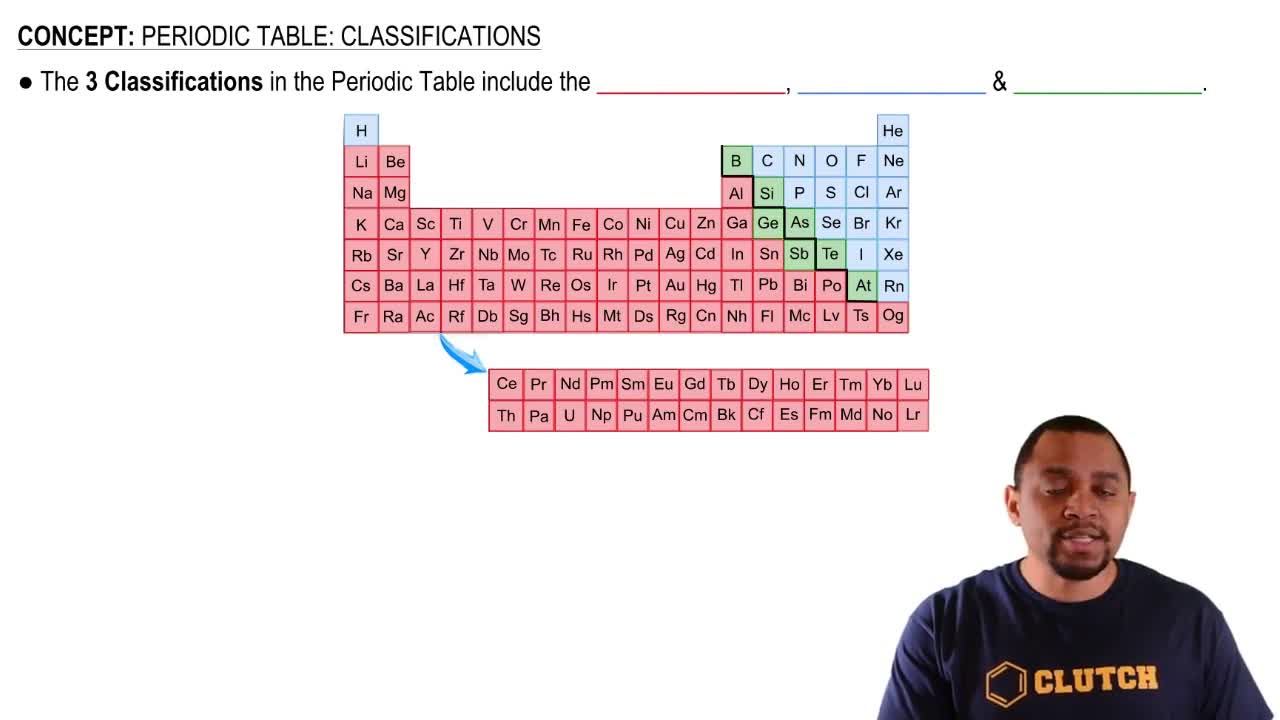
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
e. located in Group 8A (18)
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
a. located in Group 2A (2)
Using TABLE 4.4, identify the function of each of the following in the body and classify each as an alkali metal, an alkaline earth metal, a transition element, or a halogen:
b. Cu