What type of interactions between an enzyme and its substrate help to stabilize ES?

A substrate is held in the active site of an enzyme by attractive forces between the substrate and the amino acid side chains. For the outlined regions A, B, and C on the following substrate molecule:
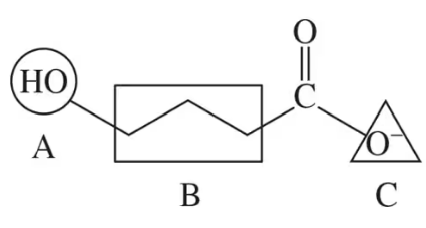
b. Could the amino acids serine, lysine, or glutamate be present in the active site? Support your answer.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Enzyme-Substrate Interaction
Amino Acid Properties

Active Site Specificity

Does each of the following statements describe a simple enzyme (no cofactor or coenzyme necessary), an enzyme that requires a cofactor, or an enzyme that requires a coenzyme?
b. consists of one polypeptide chain in its active form
Does each of the following statements describe a simple enzyme (no cofactor or coenzyme necessary), an enzyme that requires a cofactor, or an enzyme that requires a coenzyme?
c. contains vitamin B6 in its active site
If each of the following amino acid side chains is present in the active site of an enzyme, indicate whether it would (a) serve a catalytic function, (b) serve to hold the substrate, or (c) both.
a. aspartate
If each of the following amino acid side chains is present in the active site of an enzyme, indicate whether it would (a) serve a catalytic function, (b) serve to hold the substrate, or (c) both.
d. lysine
Pepsin, an enzyme that hydrolyzes peptide bonds in proteins, functions in the stomach at a pH optimum of 1.5 to 2.0. How is the rate of a pepsin-catalyzed reaction affected by each of the following conditions?
a. increasing the concentration of proteins
