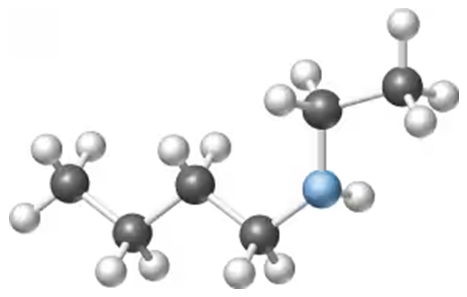
Provide compounds that fit the following descriptions:
a. Two amines that are gases at room temperature
b. A heterocyclic amine
c. A compound with an amine group on an aromatic ring
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:26m
1:26mMaster Amine Classification Concept 1 with a bite sized video explanation from Jules
Start learning