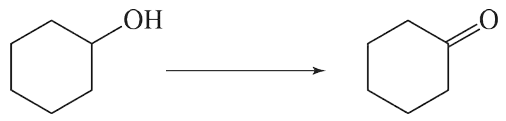
Textbook Question
Write the products for the following hydrogenation reactions:
(c)
673
views

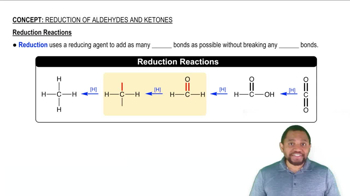
 Verified step by step guidance
Verified step by step guidance

Write the products for the following hydrogenation reactions:
(c)
Write the products for the following hydrogenation reactions:
(c)
Identify the main organic reaction shown as condensation, hydrolysis, oxidation, or reduction:
(b)
Write the products of the following reactions:
(b)
Write the products of the following reactions:
(a)
Fill in the missing organic products or reactants for the following hydrogenation reactions:
(a)