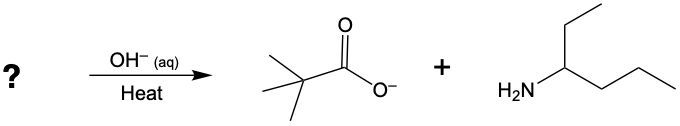
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following:
b.
 Verified step by step guidance
Verified step by step guidance
 1:12m
1:12mMaster Acidic Hydrolysis Concept 1 with a bite sized video explanation from Jules
Start learning
