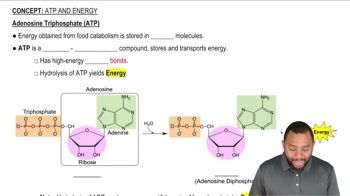
Acetyl phosphate, whose structure is given here, is another compound with a relatively high free energy of hydrolysis.
Using structural formulas, write the equation for the hydrolysis of this phosphate.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:46m
1:46mMaster Structure of Mitochondria Concept 1 with a bite sized video explanation from Jules
Start learning