Textbook Question
Draw the structures of an aldopentose.
1157
views

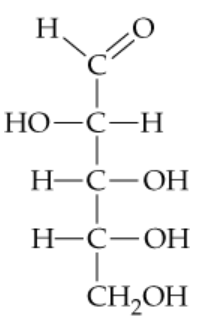
 Verified step by step guidance
Verified step by step guidance



Draw the structures of an aldopentose.
Draw the structures of a ketohexose.
Aldoheptoses have five chiral carbon atoms. What is the maximum possible number of aldoheptose stereoisomers? Draw all of the aldoheptose stereoisomers.
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
b.
D-Talose, a constituent of certain antibiotics, has the open-chain structure shown next. Draw d-talose in its cyclic hemiacetal form.
The cyclic structure of D-idose, an aldohexose, is shown in the margin. Convert this to the straight-chain Fischer projection structure.