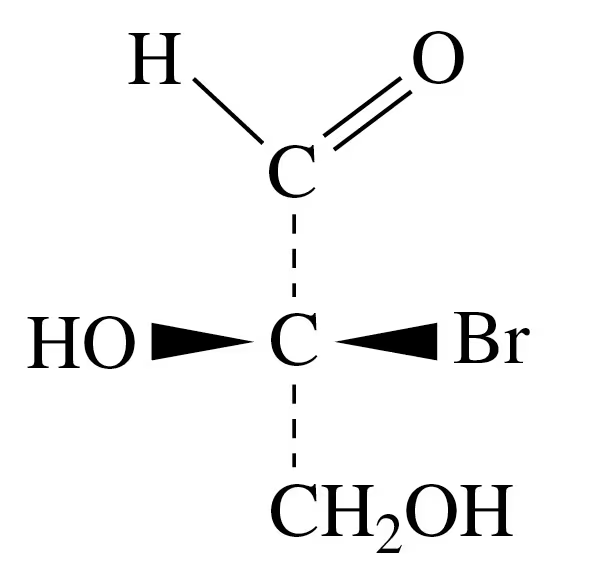
How many chiral carbon atoms are present in each of the molecules shown in Problem 20.31?
a.
b.
c.
d.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:46m
1:46mMaster Fischer Projections Concept 1 with a bite sized video explanation from Jules
Start learning