Textbook Question
Calculate the pH of each solution given the following:
c. [OH-] = 1 × 10-5 M
867
views
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: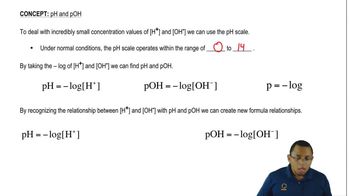



 1:31m
1:31mMaster The pH Scale Concept 1 with a bite sized video explanation from Jules
Start learning