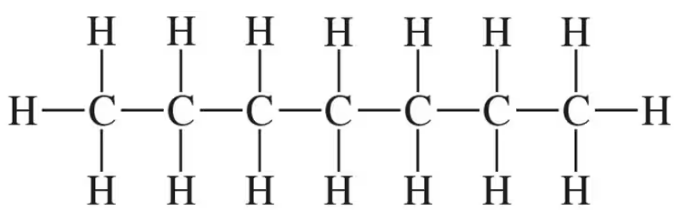
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
e. CH3―CH2―CH2―CH2―CH3
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:53m
1:53mMaster Condensed Formula Concept 1 with a bite sized video explanation from Jules
Start learning