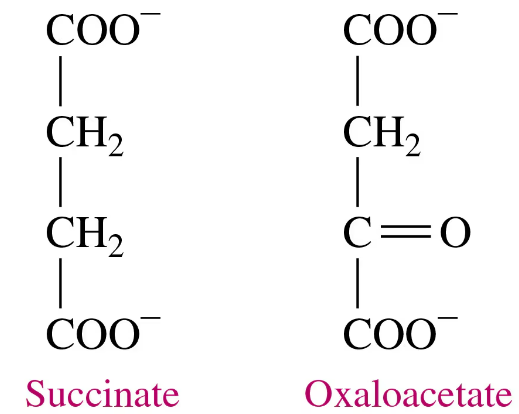
Indicate whether each of the following describes a competitive or a noncompetitive enzyme inhibitor:
a. The inhibitor has a structure similar to the substrate.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:19m
1:19mMaster Enzyme Inhibition Concept 1 with a bite sized video explanation from Jules
Start learning