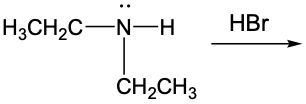
Complete the following equations. (Hint: Remember that a nitrogen with three groups bound to it has a lone pair and one with four does not)
a.
 Verified step by step guidance
Verified step by step guidance
 1:32m
1:32mMaster Acid-Base Reaction Concept 1 with a bite sized video explanation from Jules
Start learning
