Sketch the three-dimensional shape of the following molecules:
a. Methylamine, CH3NH2

 Verified step by step guidance
Verified step by step guidance

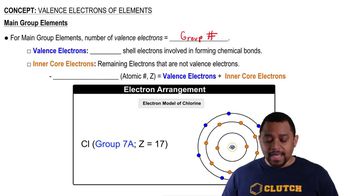

Sketch the three-dimensional shape of the following molecules:
a. Methylamine, CH3NH2
Based on electronegativity differences, would you expect bonds between the following pairs of atoms to be largely ionic or largely covalent?
b. Ca and Cl
The discovery in the 1960s that xenon and fluorine react to form a molecular compound was a surprise to most chemists, because it had been thought that noble gases could not form bonds.
a. Why was it thought that noble gases could not form bonds?
Which of the following compounds contain ionic bonds? Which contain covalent bonds? Which contain coordinate covalent bonds? (A compound may contain more than one type of bond.)
a. BaCl2
The phosphonium ion, PH4+ is formed by reaction of phosphine, PH3, with an acid.
b. Predict its molecular geometry.
The phosphonium ion, PH4+ is formed by reaction of phosphine, PH3, with an acid.
d. Explain why the ion has a +1 charge.