Textbook Question
What is the activation energy for a reaction? Why is activation energy necessary?
1573
views
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: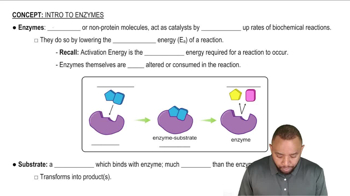



 2:14m
2:14mMaster Intro to Enzymes Concept 1 with a bite sized video explanation from Jules
Start learning