Textbook Question
Identify the metabolic nucleotide described by the following:
a. contains the vitamin riboflavin
873
views

 Verified step by step guidance
Verified step by step guidance
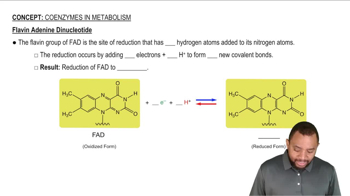

Identify the metabolic nucleotide described by the following:
a. contains the vitamin riboflavin
Identify the metabolic nucleotide described by the following:
a. contains a form of the vitamin niacin
Identify the metabolic nucleotide described by the following:
a. exchanges energy when a phosphate bond is hydrolyzed
Name a carbohydrate (if any) that undergoes digestion in each of the following sites:
a. mouth
Name a carbohydrate (if any) that undergoes digestion in each of the following sites:
c. small intestine
Describe how cholesterol is packaged after absorption in the intestine.