Textbook Question
Identify each of the following as catabolic or anabolic:
a. digestion of fats to fatty acids and glycerol
755
views

 Verified step by step guidance
Verified step by step guidance
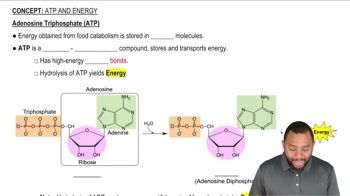

Identify each of the following as catabolic or anabolic:
a. digestion of fats to fatty acids and glycerol
Identify each of the following as catabolic or anabolic:
c. synthesis of nucleic acids from nucleotides
What is the purpose of digestion in stage 1?
What is the role of bile salts in lipid digestion?
How are insoluble triacylglycerols transported to the cells?