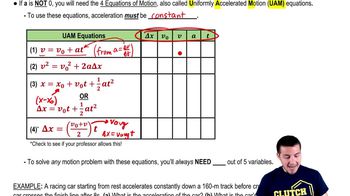
Estimate how many air molecules rebound from a wall in a typical room per second, assuming an ideal gas of N molecules contained in a cubic room with sides of length ℓ at temperature T and pressure P.
(a) Show that the frequency f with which gas molecules strike a wall is ƒ = ( /2)(P/kT) ℓ² where is the average x component of the molecule’s velocity.
(b) Show that the equation can then be written as ƒ≈ Pℓ² / where m is the mass of a gas molecule.