Multiple Choice
What do the sign and magnitude of the ΔG of a reaction tell us about the speed of the reaction? and
3124
views
1
rank
 Verified step by step guidance
Verified step by step guidance
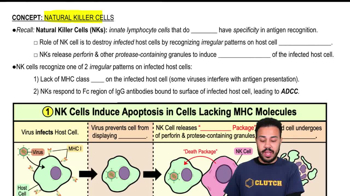


 1:56m
1:56mMaster Chemical Reactions with a bite sized video explanation from Bruce Bryan
Start learning