Explain how feedback inhibition regulates metabolic pathways.

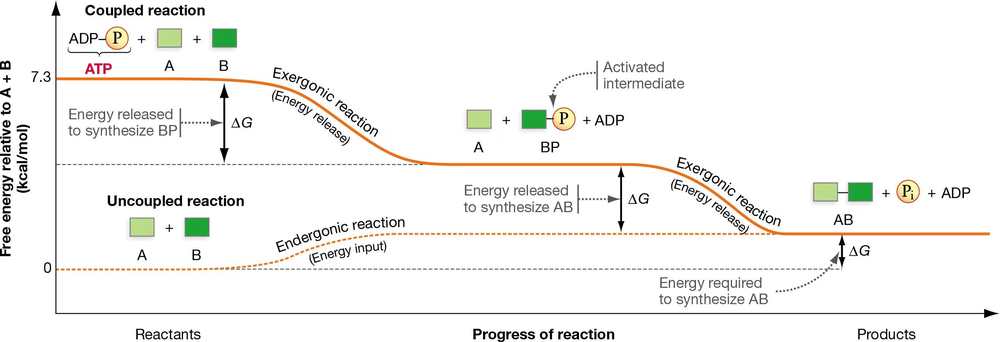
In Figure 8.10, the energetic coupling of substrate phosphorylation and an endergonic reaction are shown. If the hydrolysis of ATP releases 7.3 kcal of free energy, use the graph in this figure to estimate what you would expect the ∆G values to be for the uncoupled reaction and the two steps in the coupled reaction.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Energetic Coupling

Gibbs Free Energy (∆G)

Substrate Phosphorylation
Explain the lock-and-key model of enzyme activity. What is incorrect about this model?
If you were to expose glucose to oxygen on your lab bench, why would you not expect to see it burn as described by the reaction in Figure 8.6?
a. The reaction is endergonic and requires an input of energy.
b. The reaction is not spontaneous unless an enzyme is added.
c. The sugar must first be phosphorylated to increase its potential energy.
d. Activation energy is required for the sugar and oxygen to reach their transition state.
Using what you have learned about changes in Gibbs free energy, would you predict the ∆G value of catabolic reactions to be positive or negative? What about anabolic reactions? Justify your answers using the terms 'enthalpy' and 'entropy.'
Draw a chemical equation to represent the redox reaction that occurs when methane (CH4) burns in the presence of oxygen (O2). Identify the reactant that is reduced and the reactant that is oxidized. Of the four molecules that should be in your equation, point out the one that has bonds with the highest potential energy.
You have discovered an enzyme that appears to function only when a particular sugar accumulates. Which of the following scenarios would you predict to be responsible for activating this enzyme?
a. The sugar cleaves the enzyme to form the active conformation.
b. The sugar is an allosteric regulatory molecule for the enzyme.
c. The sugar is a competitive inhibitor for the enzyme.
d. The sugar phosphorylates the enzyme to form the active conformation.
