Textbook Question
Fill in the blanks in this concept map to help you tie together the key concepts concerning elements, atoms, and molecules.
3310
views

 Verified step by step guidance
Verified step by step guidance
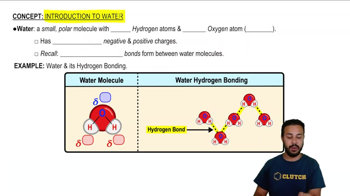
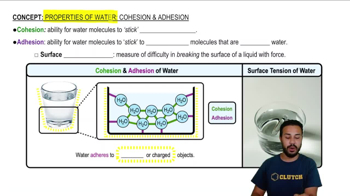

Fill in the blanks in this concept map to help you tie together the key concepts concerning elements, atoms, and molecules.
Changing the _________ would change it into an atom of a different element.
a. Number of electrons surrounding the nucleus of an atom
b. Number of protons in the nucleus of an atom
c. Electrical charge of an atom
d. Number of neutrons in the nucleus of an atom
What is chemically nonsensical about this structure?
A solution at pH 6 contains _________ H+ than the same amount of a solution at pH 8.
a. 20 times more
b. 100 times more
c. 2 times less
d. 100 times less