Multiple Choice
Which statement is true for a cooling curve of a pure substance?
43
views
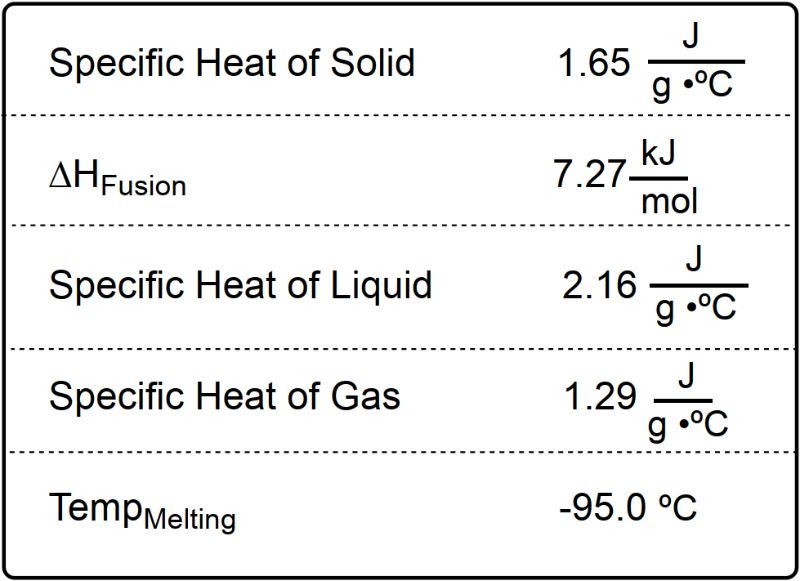
-11.406 kJ
-39.820 kJ
-22.811 kJ
-82.592 kJ
 Verified step by step guidance
Verified step by step guidance
 3:52m
3:52mMaster Introduction to Heating and Cooling Curves with a bite sized video explanation from Jules
Start learning
