 Back
BackProblem 74
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. F b. Sr c. K d. Ne e. At
Problem 76
Which pair of elements do you expect to be most similar? Why? a. nitrogen and oxygen b. titanium and gallium c. lithium and sodium d. germanium and arsenic e. argon and bromine
- How would you sketch the mass spectrum of gallium given its two naturally occurring isotopes with the following masses and natural abundances: Isotope Mass (amu) Abundance (%) Ga-69 68.92558 60.108 Ga-71 70.92470 39.892?
Problem 79
Problem 81
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.
- The atomic mass of copper is 63.546 amu. Do any copper isotopes have an exact mass of 63.546 amu? Explain.
Problem 82
Problem 83
An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. Find the atomic mass of this element and identify it.
Problem 84
An element has four naturally occurring isotopes with the masses and natural abundances given here. Find the atomic mass of the element and identify it.
Isotope Mass (amu) Abundance (%)
1 135.90714 0.19
2 137.90599 0.25
3 139.90543 88.43
4 141.90924 11.13
Problem 85b
Bromine has two naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904 amu. The mass of Br-81 is 80.9163 amu, and its natural abundance is 49.31%. Calculate the mass and natural abundance of Br-79.
Problem 86
Silicon has three naturally occurring isotopes (Si-28, Si-29, and Si-30). The mass and natural abundance of Si-28 are 27.9769 amu and 92.2%, respectively. The mass and natural abundance of Si-29 are 28.9765 amu and 4.67%, respectively. Find the mass and natural abundance of Si-30.
Problem 87
Use the mass spectrum of europium to determine the atomic mass of europium.
Problem 88
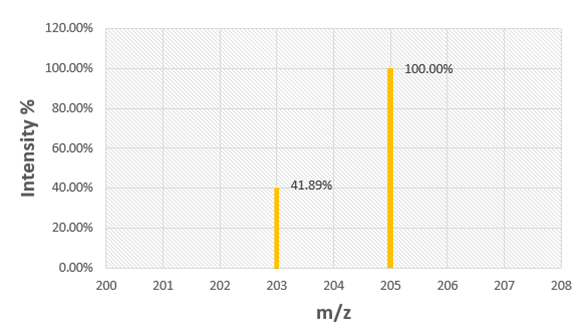
Use the mass spectrum of rubidium to determine the atomic mass of rubidium.
Problem 91
How many sulfur atoms are there in 5.52 mol of sulfur?
Problem 92
A gold sample contains 4.65×1024 gold atoms. How many moles of gold does the sample contain?
Problem 93c
What is the amount, in moles, of each elemental sample? a. 23.2 g Kr
Problem 94a
What is the mass, in grams, of each elemental sample? a. 0.0133 mol Xe
Problem 94c
What is the mass, in grams, of each elemental sample? c. 1.75×10-3 mol Hg
Problem 95
How many silver atoms are there in 9.55 g of silver?
Problem 96
What is the mass of 4.51×1022 Pb atoms?
Problem 97
Calculate the number of atoms in each sample. a. 5.18 g P
Problem 98
Calculate the number of atoms in each sample. a. 14.955 g Cr
Problem 99a,b
Calculate the mass, in grams, of each sample. a. 1.1×1023 gold atoms b. 2.82×1022 helium atoms
Problem 99c
Calculate the mass, in grams, of each sample. c. 1.8×1023 lead atoms
Problem 99d
Calculate the mass, in grams, of each sample. d. 7.9×1021 uranium atoms
Problem 100
Calculate the mass, in kg, of each sample. a. 7.55×1026 cadmium atoms b. 8.15×1027 nickel atoms c. 1.22×1027 manganese atoms d. 5.48×1029 lithium atoms
Problem 101
How many carbon atoms are there in a diamond (pure carbon) with a mass of 83 mg?
Problem 102
How many helium atoms are there in a helium blimp containing 587 kg of helium?
Problem 103
Calculate the average mass, in grams, of one platinum atom.
Problem 104
Using scanning tunneling microscopy, scientists at IBM wrote the initials of their company with 35 individual xenon atoms (as shown below). Calculate the total mass of these letters in grams.
Problem 105
A 7.83 g sample of HCN contains 0.290 g of H and 4.06 g of N. Find the mass of carbon in a sample of HCN with a mass of 3.37 g.
Problem 106
The ratio of sulfur to oxygen by mass in SO2 is 1.0:1.0. a. Find the ratio of sulfur to oxygen by mass in SO3. b. Find the ratio of sulfur to oxygen by mass in S2O.
