 Back
BackProblem 55b
Write isotopic symbols in the form AZX for each isotope. b. the iron isotope with 30 neutrons
Problem 55c
Write isotopic symbols in the form AZX for each isotope. c. the rubidium isotope with 45 neutrons
Problem 57a
Determine the number of protons and the number of neutrons in each isotope. a. 147N
Problem 57d
Determine the number of protons and the number of neutrons in each isotope. d. 20882Pb
Problem 58
Determine the number of protons and the number of neutrons in each isotope. a. 4019K b. 22688Ra c. 9943Tc d. 3315P
- Determine the number of protons and the number of neutrons in carbon-14 and write its symbol in the form AZX, considering that the amount of carbon-14 in ancient artifacts and fossils is often used to establish their age.
Problem 59
Problem 61
Determine the number of protons and the number of electrons in each ion. a. Ni2+ b. S2– c. Br– d. Cr3+
Problem 62b
Determine the number of protons and the number of electrons in each ion. b. Se2-
Problem 62c
Determine the number of protons and the number of electrons in each ion. c. Ga3+
Problem 62d
Determine the number of protons and the number of electrons in each ion. d. Sr2+
Problem 64a
Predict the charge of the ion formed by each element. a. Sr
Problem 64c
Predict the charge of the ion formed by each element. c. I
Problem 66
Fill in the blanks to complete the table.
Symbol Ion Formed Number of Electrons in Ion Number of Protons in Ion
Cl ______ ______ 17
Te ______ 54 ______
Br Br– ______ ______
______ Sr2+ ______ 38
Problem 70
Write the symbol for each element and classify it as a metal, nonmetal, or metalloid. a. bromine b. potassium c. lead d. silicon e. silver
Problem 71
Determine whether or not each element is a main-group element. a. tellurium b. potassium c. vanadium d. manganese
Problem 72
Determine whether or not each element is a transition element. a. Cr b. Br c. Mo d. Cs
Problem 74
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. F b. Sr c. K d. Ne e. At
Problem 76
Which pair of elements do you expect to be most similar? Why? a. nitrogen and oxygen b. titanium and gallium c. lithium and sodium d. germanium and arsenic e. argon and bromine
- How would you sketch the mass spectrum of gallium given its two naturally occurring isotopes with the following masses and natural abundances: Isotope Mass (amu) Abundance (%) Ga-69 68.92558 60.108 Ga-71 70.92470 39.892?
Problem 79
Problem 81
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.
- The atomic mass of copper is 63.546 amu. Do any copper isotopes have an exact mass of 63.546 amu? Explain.
Problem 82
Problem 83
An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. Find the atomic mass of this element and identify it.
Problem 84
An element has four naturally occurring isotopes with the masses and natural abundances given here. Find the atomic mass of the element and identify it.
Isotope Mass (amu) Abundance (%)
1 135.90714 0.19
2 137.90599 0.25
3 139.90543 88.43
4 141.90924 11.13
Problem 85b
Bromine has two naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904 amu. The mass of Br-81 is 80.9163 amu, and its natural abundance is 49.31%. Calculate the mass and natural abundance of Br-79.
Problem 86
Silicon has three naturally occurring isotopes (Si-28, Si-29, and Si-30). The mass and natural abundance of Si-28 are 27.9769 amu and 92.2%, respectively. The mass and natural abundance of Si-29 are 28.9765 amu and 4.67%, respectively. Find the mass and natural abundance of Si-30.
Problem 87
Use the mass spectrum of europium to determine the atomic mass of europium.
Problem 88
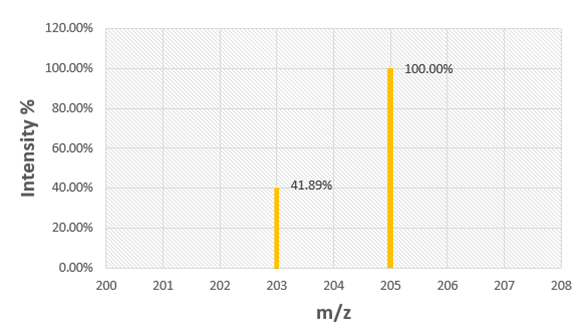
Use the mass spectrum of rubidium to determine the atomic mass of rubidium.
Problem 91
How many sulfur atoms are there in 5.52 mol of sulfur?
Problem 92
A gold sample contains 4.65×1024 gold atoms. How many moles of gold does the sample contain?
Problem 93c
What is the amount, in moles, of each elemental sample? a. 23.2 g Kr
