The human genome is 3×10⁹ bp. You wish to design a primer to amplify a specific gene in the genome. In general, what length of oligonucleotide would be sufficient to amplify a single unique sequence? To simplify your calculation, assume that all bases occur with an equal frequency.
Table of contents
- 1. Introduction to Genetics51m
- 2. Mendel's Laws of Inheritance3h 37m
- 3. Extensions to Mendelian Inheritance2h 41m
- 4. Genetic Mapping and Linkage2h 28m
- 5. Genetics of Bacteria and Viruses1h 21m
- 6. Chromosomal Variation1h 48m
- 7. DNA and Chromosome Structure56m
- 8. DNA Replication1h 10m
- 9. Mitosis and Meiosis1h 34m
- 10. Transcription1h 0m
- 11. Translation58m
- 12. Gene Regulation in Prokaryotes1h 19m
- 13. Gene Regulation in Eukaryotes44m
- 14. Genetic Control of Development44m
- 15. Genomes and Genomics1h 50m
- 16. Transposable Elements47m
- 17. Mutation, Repair, and Recombination1h 6m
- 18. Molecular Genetic Tools19m
- 19. Cancer Genetics29m
- 20. Quantitative Genetics1h 26m
- 21. Population Genetics50m
- 22. Evolutionary Genetics29m
18. Molecular Genetic Tools
Genetic Cloning
Problem 7a
Textbook Question
Using animal models of human diseases can lead to insights into the cellular and genetic bases of the diseases. Duchenne muscular dystrophy (DMD) is the consequence of an X-linked recessive allele.
How would you make a mouse model of DMD?
 Verified step by step guidance
Verified step by step guidance1
Understand the genetic basis of Duchenne muscular dystrophy (DMD): DMD is caused by mutations in the dystrophin gene, which is located on the X chromosome. This gene encodes the dystrophin protein, essential for muscle function. Since DMD is X-linked recessive, males with one mutated allele exhibit the disease, while females require two mutated alleles to show symptoms.
Identify the mouse dystrophin gene: The mouse genome contains a homologous dystrophin gene similar to the human dystrophin gene. This gene must be targeted to create a model of DMD.
Use gene-editing techniques to introduce mutations: Employ a gene-editing tool such as CRISPR-Cas9 to introduce a specific mutation into the dystrophin gene in mouse embryonic stem cells. The mutation should mimic the type of mutation found in human DMD patients, such as a frameshift or nonsense mutation that disrupts dystrophin production.
Generate genetically modified mice: Inject the edited embryonic stem cells into mouse blastocysts and implant them into surrogate mothers. The resulting offspring will carry the targeted mutation in the dystrophin gene. Breed these mice to produce homozygous mutants (females with two mutated alleles or males with one mutated allele) to study the disease phenotype.
Validate the mouse model: Confirm the absence of dystrophin protein in the muscle tissue of the genetically modified mice using techniques such as Western blotting or immunohistochemistry. Assess the mice for symptoms of DMD, such as muscle weakness and degeneration, to ensure the model accurately reflects the human disease.
 Verified video answer for a similar problem:
Verified video answer for a similar problem:This video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
X-linked Recessive Inheritance
X-linked recessive inheritance refers to a pattern of genetic transmission where a gene located on the X chromosome is expressed in males (who have one X and one Y chromosome) if they inherit the recessive allele. In females, who have two X chromosomes, the presence of one normal allele can mask the effect of the recessive allele. This is crucial for understanding diseases like Duchenne muscular dystrophy (DMD), which predominantly affects males.
Recommended video:
Guided course
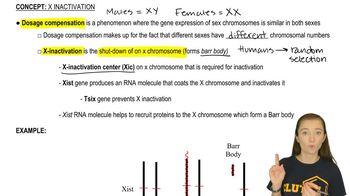
X-Inactivation
Animal Models in Genetics
Animal models, such as mice, are used in genetics to study human diseases by replicating the genetic and phenotypic characteristics of the condition. These models allow researchers to investigate disease mechanisms, test potential treatments, and understand the genetic basis of disorders. Creating a mouse model for DMD involves manipulating the mouse genome to mimic the human mutation responsible for the disease.
Recommended video:
Guided course

Genetics of Development
Gene Editing Techniques
Gene editing techniques, such as CRISPR-Cas9, enable precise modifications to an organism's DNA. In the context of creating a mouse model for DMD, these techniques can be employed to introduce specific mutations in the mouse genome that correspond to the mutations found in human patients. This allows for the study of disease progression and the evaluation of therapeutic strategies in a controlled environment.
Recommended video:
Guided course

Mapping Genes
Related Videos
Related Practice
Textbook Question
556
views


