Textbook Question
Complete the following statements:
c. Sodium and potassium are examples of elements called _____.
43
views

 Verified step by step guidance
Verified step by step guidance
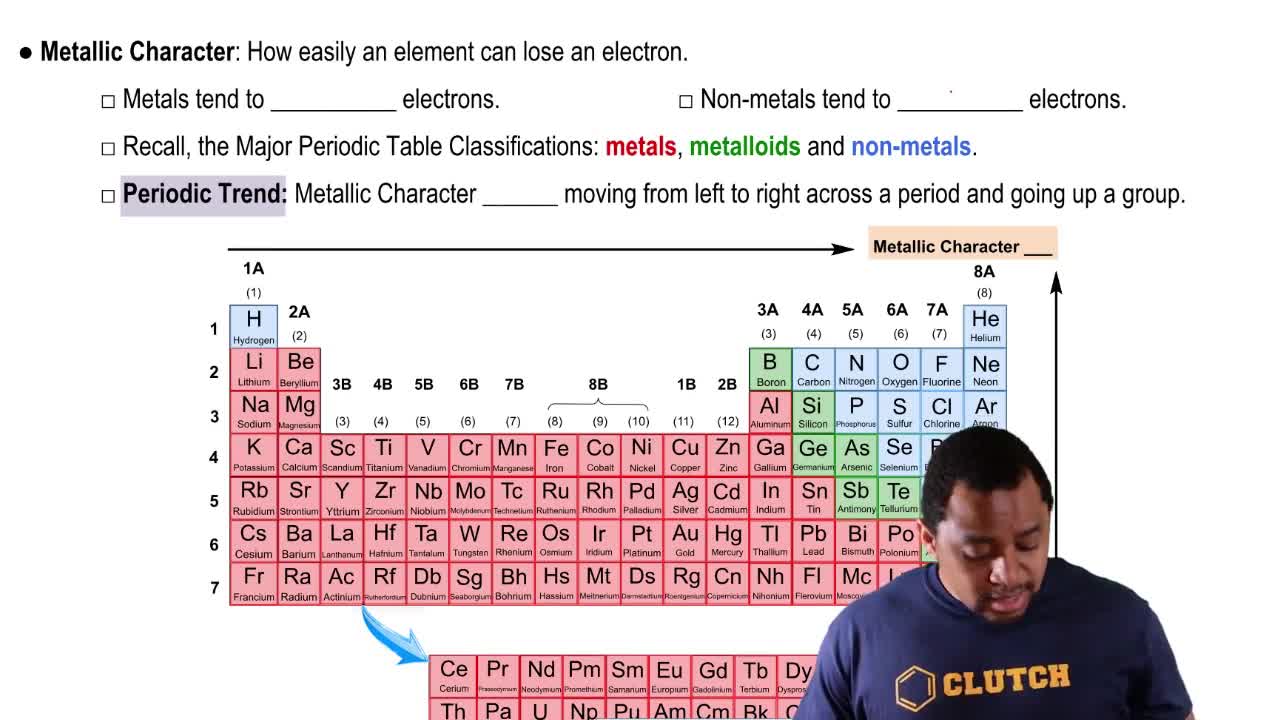
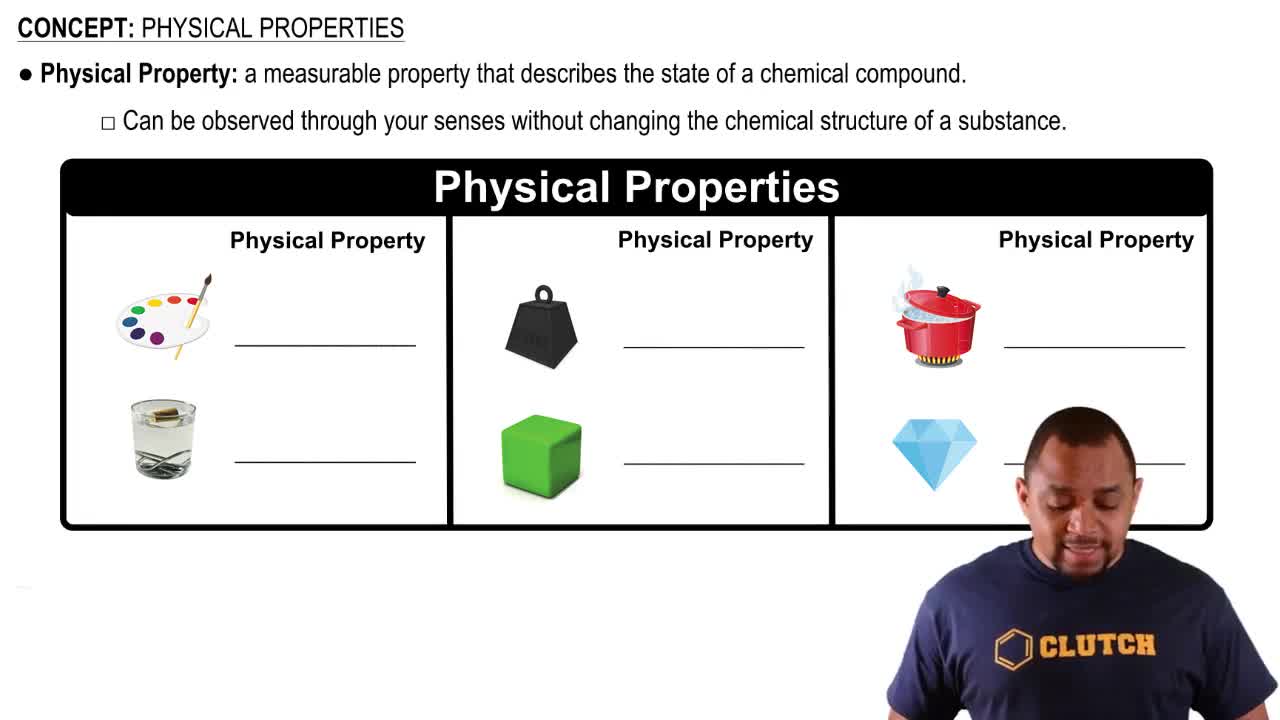
Complete the following statements:
c. Sodium and potassium are examples of elements called _____.
Complete the following statements:
a. The number of protons and neutrons in an atom is also the _____ number.
Complete the following statements:
b. The elements in Group 7A (17) are called the _____.
Indicate if each of the following statements is true or false:
c. The atomic mass unit is based on a carbon atom with six protons and six neutrons.
Which of the following molecules will produce the most ATP per mole?
a. glucose or stearic acid (C18)
Indicate if each of the following statements is true or false:
d. The proton and the electron have about the same mass.