Using the Chemistry Link to Health: Elements Essential to Health, answer each of the following:
b. What is the role of sulfur in the human body?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: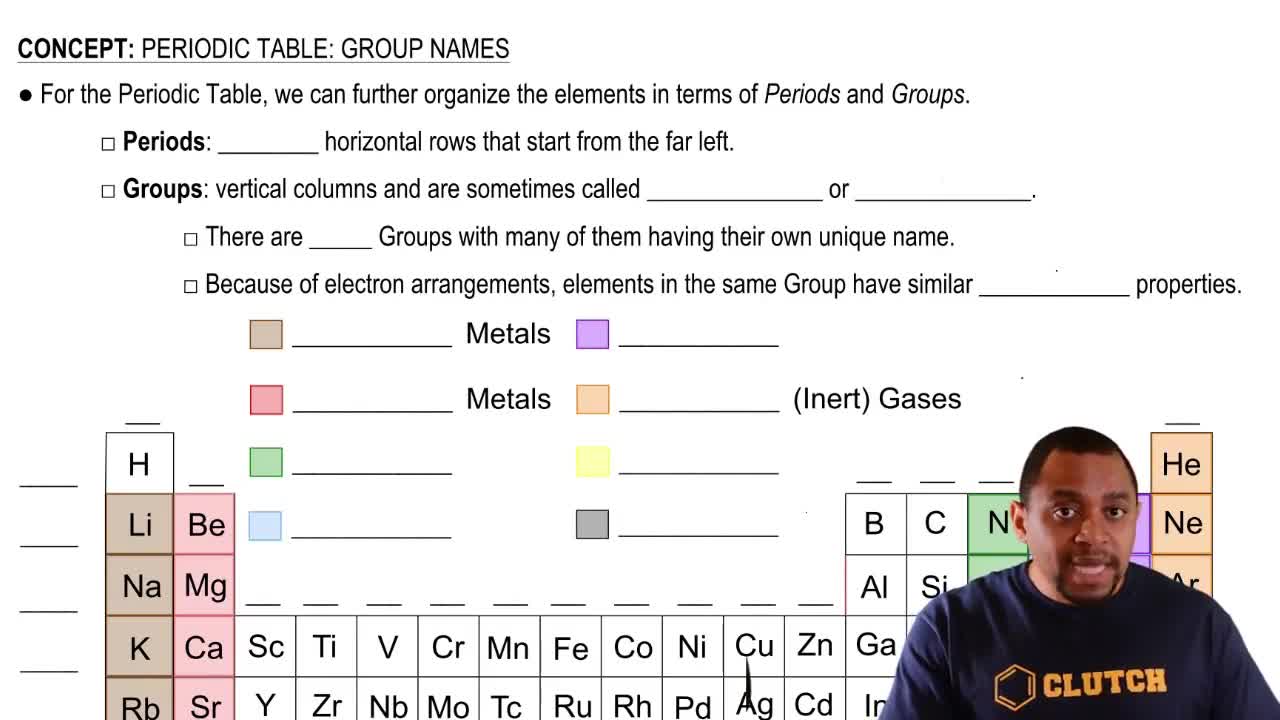

 2:40m
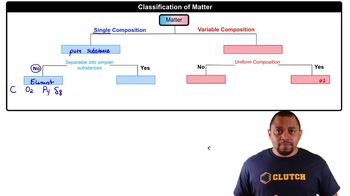
2:40mMaster Periodic Table: Classifications with a bite sized video explanation from Jules
Start learning