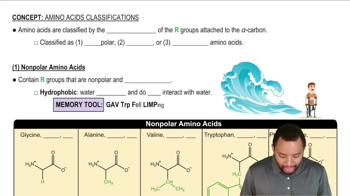
What type of interaction would you expect between the side chains of each of the following pairs of amino acids in the tertiary structure of a protein?
b. alanine and valine
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:7m
2:7mMaster Tertiary Protein Structure Concept 1 with a bite sized video explanation from Jules
Start learning