Textbook Question
Write the formula for the conjugate base for each of the following acids:
c. HPO42-
798
views

 Verified step by step guidance
Verified step by step guidance

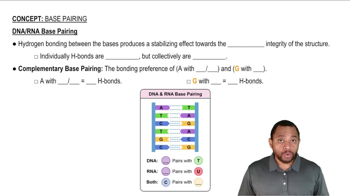

Write the formula for the conjugate base for each of the following acids:
c. HPO42-
Write the formula for the conjugate acid for each of the following bases:
a. CO32-
Write the formula for the conjugate acid for each of the following bases:
c. H2PO4-
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
a. HBr or HNO2
Using TABLE 10.3, identify the stronger acid in each of the following pairs:
b. H3PO4 or HSO4-
What is meant by the term reversible reaction?