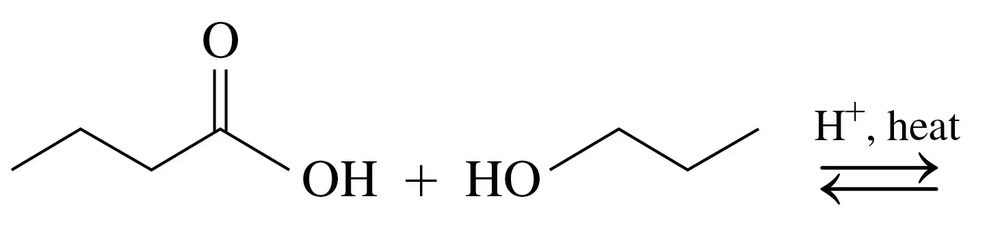
Give the structure of the repeating units in the polymers that are formed in the reactions of the following compounds.
a.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:5m
1:5mMaster Ester Reactions: Esterification Concept 1 with a bite sized video explanation from Jules
Start learning