Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Charge
Ionic charge refers to the electrical charge that an ion carries, which results from the loss or gain of electrons. In the case of Sr²⁺, the '²⁺' indicates that the strontium ion has lost two electrons, giving it a positive charge. Understanding ionic charge is essential for naming ions and predicting their behavior in chemical reactions.
Recommended video:
Cation
A cation is a positively charged ion formed when an atom loses one or more electrons. Strontium (Sr) typically forms a cation with a charge of +2, denoted as Sr²⁺. Recognizing the difference between cations and anions (negatively charged ions) is crucial for understanding ionic compounds and their properties.
Recommended video:
Naming Monoatomic Cations Concept 2
Nomenclature of Ions
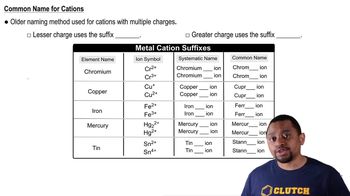
Nomenclature of ions involves the systematic naming of ions based on their charge and the elements they derive from. For example, the name for Sr²⁺ is 'strontium ion.' Familiarity with the rules of nomenclature helps in accurately identifying and communicating about various ions in chemistry.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:24m
1:24m