Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Charge
Ionic charge refers to the electrical charge that an ion carries, which is determined by the loss or gain of electrons. In the case of Au³⁺, the '³⁺' indicates that the gold ion has lost three electrons, resulting in a positive charge. Understanding ionic charges is essential for naming ions correctly, as it informs the oxidation state of the element.
Recommended video:
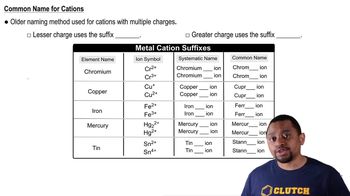
Roman Numerals in Nomenclature
Roman numerals are used in chemical nomenclature to indicate the oxidation state of transition metals in compounds. For example, in the name 'gold(III)', the Roman numeral 'III' signifies that gold has a +3 oxidation state. This practice is crucial for distinguishing between different ionic forms of the same element, especially for metals that can exhibit multiple oxidation states.
Recommended video:
Naming Monoatomic Cations Concept 1
Naming Cations
Naming cations involves identifying the element and its charge. For transition metals like gold, the name of the element is followed by the oxidation state in Roman numerals. In the case of Au³⁺, the correct name is 'gold(III)', which reflects both the identity of the ion and its positive charge, ensuring clarity in chemical communication.
Recommended video:
Naming Monoatomic Cations Concept 2
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:24m
1:24m