Textbook Question
Determine whether each of the following chemical equations is balanced or not balanced:
d.
900
views

 Verified step by step guidance
Verified step by step guidance
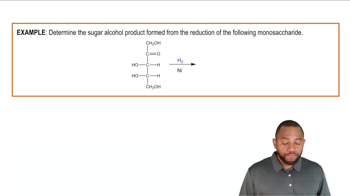


Determine whether each of the following chemical equations is balanced or not balanced:
d.
Balance each of the following chemical equations:
c. Sb2S3(s) + HCl(aq) → SbCl3(aq) + H2S(g)
Balance each of the following chemical equations:
d. Al(s) + HCl(aq) → H2(g) + AlCl3(aq)
In the mitochondria of human cells, energy is provided by the oxidation and reduction reactions of the iron ions in the cytochromes in electron transport. Identify each of the following as an oxidation or a reduction:
a. Fe3+ + e– → Fe2+
The chemical reaction of hydrogen with oxygen produces water.
2 H2(g) + O2(g) → 2 H2O(g)
c. How many moles of H2O form when 2.5 moles of O2 reacts?
Why do chemical reactions require energy of activation?