Why is it not possible to balance an equation by changing the subscript on a substance, say from H2O to H2O2?

In each of the following, tell whether the substance gains electrons or loses electrons in a redox reaction:
a. An oxidizing agent
b. A reducing agent
c. A substance undergoing oxidation
d. A substance undergoing reduction
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Oxidizing Agent
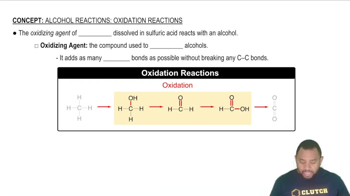
Reducing Agent

Oxidation and Reduction
Which of the following equations are balanced? Balance those that need it.
a. CaC2 + 2 H2O → Ca(OH)2 +C2H2
b. C2H8N2 + 2 N2O4 → 2 N2 + 2 CO2 + 4 H2O
c. 3 MgO + 2 Fe → Fe2O3 + 3 Mg
d. N2O → N2 + O2
When sodium metal is placed in water, the following change occurs: Sodium, Na(s) + Water, H2O(l) → Hydrogen, H2(g) + Sodium hydroxide, NaOH(aq)
a. Identify the reactants and products and their physical states
High temperature combustion processes, such as in combustion engines and coal-fired power plants, can result in the reaction of nitrogen and sulfur with oxygen to form nitrogen oxides (NO𝓍) and sulfur oxides (SO𝓍), where x can vary. These NO𝓍 and SO𝓍 compounds subsequently undergo further reaction in the atmosphere to create acidic compounds that contribute to acid rain.
a. Do some research to determine the common products that are formed (i.e., what are the values of x) for the reactions of N and S with oxygen. Write balanced equations for these reactions.
Many pharmaceuticals are marketed with the designation "HCl" appended to the name of the drug. What does the "HCl" mean? What type of reaction would be involved in converting a drug to the HCl form? What are the advantages of this form of the drug?
