Textbook Question
Indicate whether each of the following statements is characteristic of an acid, a base, or both:
a. neutralizes acids
953
views
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: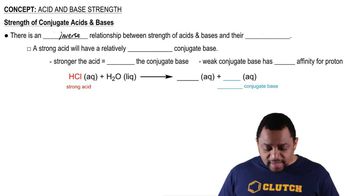
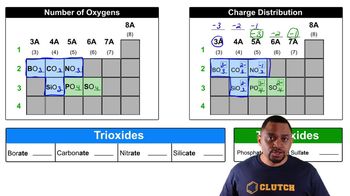
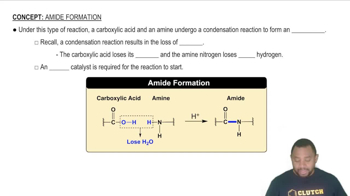

 0:40m
0:40mMaster Acid-Base Introduction Concept 1 with a bite sized video explanation from Jules
Start learning