Write the formula for the conjugate acid for each of the following bases:
a. CO32-
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: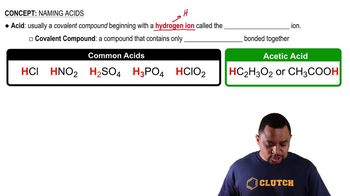

 0:40m
0:40mMaster Acid-Base Introduction Concept 1 with a bite sized video explanation from Jules
Start learning