Which of the following contains a coordinate covalent bond? (Hint: How many covalent bonds would you expect the central atom (underlined/bold) to form?)
a. PbCl2
b. Cu(NH3)42+
c. NH4+

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: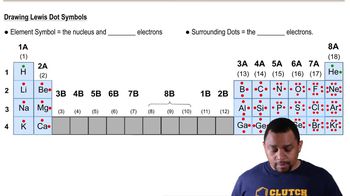



 1:10m
1:10mMaster Covalent Bonds Concept 1 with a bite sized video explanation from Jules
Start learning