Identify the bonds formed between the following pairs of atoms as either covalent or ionic.
d. Zinc and fluorine

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: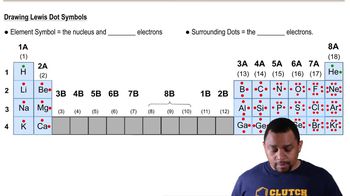



 1:10m
1:10mMaster Covalent Bonds Concept 1 with a bite sized video explanation from Jules
Start learning