Textbook Question
Give the symbol of the element described by each of the following:
a. the alkaline earth metal in Period 2
1417
views

 Verified step by step guidance
Verified step by step guidance
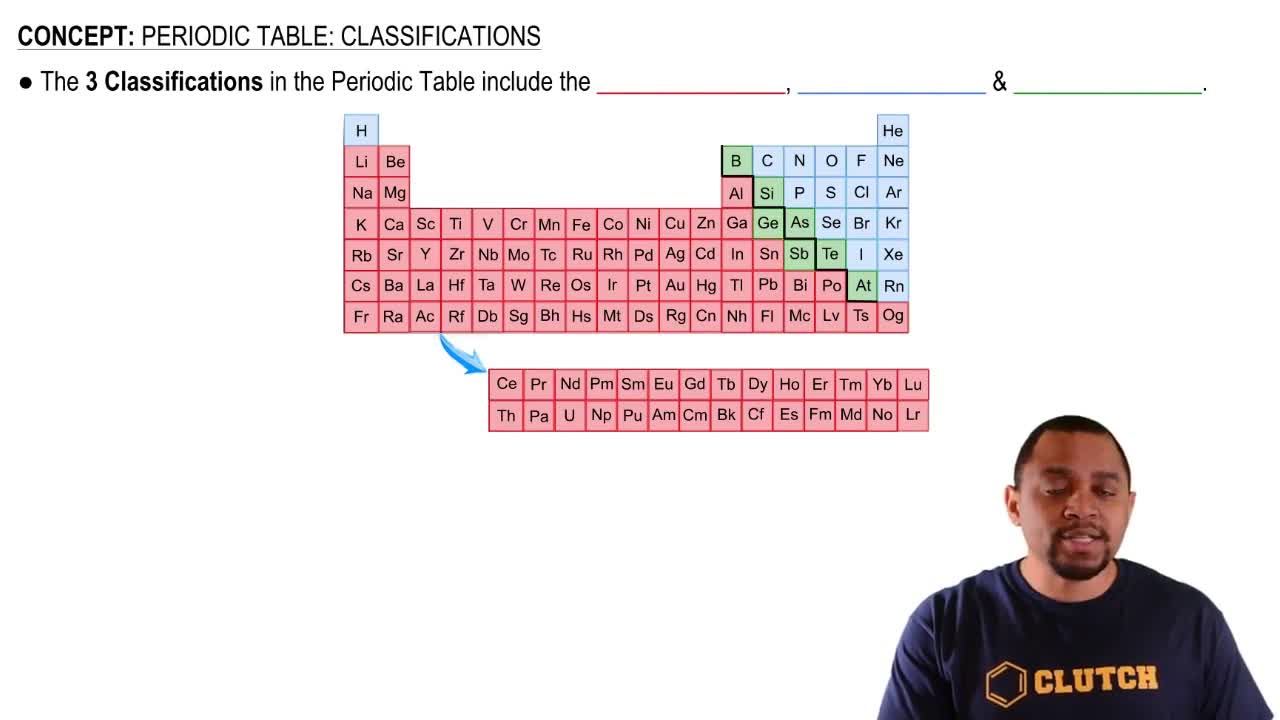
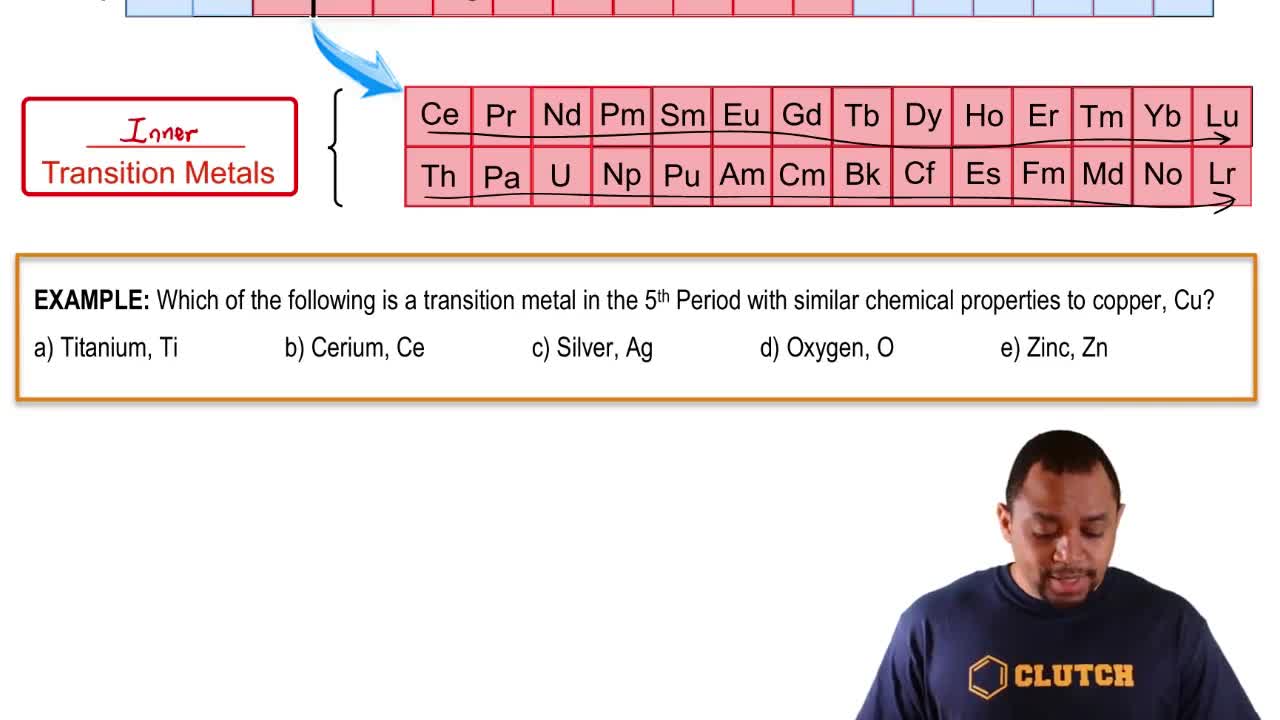
Give the symbol of the element described by each of the following:
a. the alkaline earth metal in Period 2
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
e. located in Group 8A (18)
Identify each of the following elements as a metal, a nonmetal, or a metalloid:
a. located in Group 2A (2)
Identify each of the following as describing either a proton, a neutron, or an electron:
a. has the smallest mass
Is each of the following statements true or false?
c. Neutrons repel each other.
On a dry day, your hair flies apart when you brush it. How would you explain this?